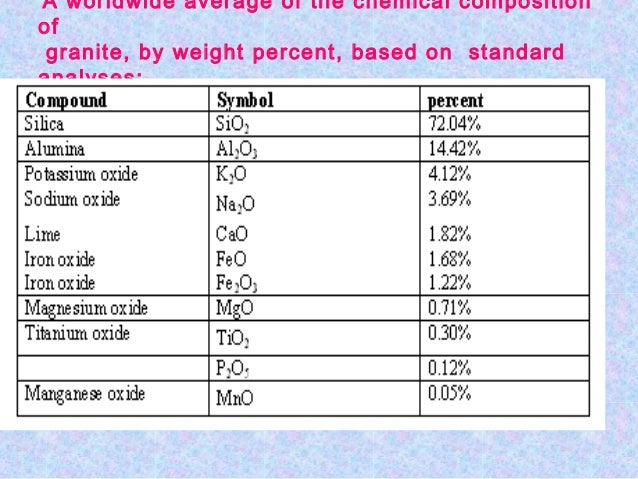
The exact chemical composition of marble will greatly vary dependong on the location and the minerals or impurities present in the limestone during recrystallization.
Marble chemical symbol.
Marble is a metamorphic rock composed of recrystallized carbonate minerals most commonly calcite or dolomite marble is typically not foliated although there are exceptions in geology the term marble refers to metamorphosed limestone but its use in stonemasonry more broadly encompasses unmetamorphosed limestone.
Heat and pressure applied to limestone creates.
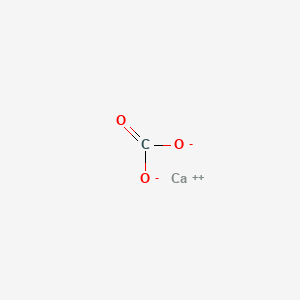
Chemical formula for marble is caco3.
Typically marble is composed of the following major constituents.
Here it means 1 calcium 1 carbon and 3 oxygen.
The only difference between limestone and marble is the crystalline structure.
The reason i think it would vary is that you get different reactions when one puts pure copper in either concentrated or dilute nitric acid.
My guess is that the answer would vary depending on the concentration of the nitric acid.
Marble is commonly used for sculpture and as a building material.
They also call marble as calcium carbonate.
It s a metamorphic rock which means that it was formed from other rocks.
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element symbols numbers and sometimes also other symbols such as parentheses dashes brackets commas and plus and minus signs.
It is used for acid neutralization in the chemical industry as well.
Marble is often crushed and used for acid neutralization in streams lakes and soils.
The marble chip is calcium carbonate caco3.
It is one of the most effective acid neutralization materials.
Marble is a type of rock.
Marble is made of calcium carbonate caco3 which is also what limestone is made from.
Limestone crystals are much smaller than that of marble and limestone is much more porous.
One for concentrated hno3 has no2 nasty nitrogen dioxide as a product while the other for dilute hno3 has not.